
About US
ABOUT US
Apurano Pharmaceuticals GmbH is a vertically integrated pharmaceutical company that specializes in the development, manufacturing and clinical testing of nanotechnological active ingredients. Due to Apurano’s strong research and innovation orientation, the company was funded by the Bavarian State Ministry for Economic Affairs and Media, Energy and Technology just one year after it was founded. This was followed by four further government research and development grants. The heart of Apurano is the globally patented PuranoTec® manufacturing technology, enabling the conversion of poorly water-soluble active ingredients from natural materials such as plants, fungi or cyanobacteria into an absorbable form. This is the only way the natural active ingredients can be effectively absorbed by the body and fully develop their effect.
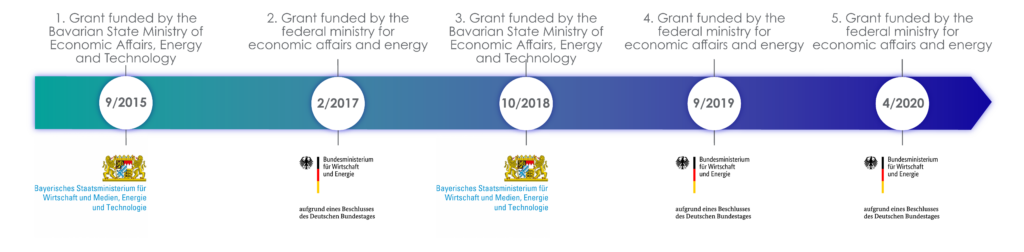
Fig.: State research and development funding for Apurano Pharmaceuticals GmbH

OUR MISSION
With the help of intensive basic research and our innovative manufacturing technology PuranoTec®, we want to use the full potential of natural active ingredients to sustainably improve the health of many people. To this end, we not only focus on our company’s ability to innovate and the use of new IT methods and tools, but also rely on the decades of experience and expertise of leading scientists and university hospitals with whom we work closely.
MANAGEMENT TEAM
Apurano Pharmaceuticals is led by a team of seasoned industry executives who collectively bring decades of productiontechnology, product development, clinical development and regulatory affairs experience to the company. The team not only shares an unparalleled determination and passion to help patients, but they also have a history of working together to successfully build companies and bring novel and effective therapies to market

Dr. Werner Brand
Managing Director
- Founder and Managing Director of Apurano Pharmaceuticals
- Entrepreneur since 2007: Founder of five pharmaceutical companies
- Inventor of five patent families and of the PuranoTec manufacturing process
- Many years of experience in management positions in companies in the automotive, process engineering and management consulting
- Studied aerospace engineering at the Technical University of Munich, The University Manchester (UK) and Northwestern University (USA), PhD at the University of Sheffield (UK)

Stefan Gähler-Brand
Chief Operating Officer
- Many years of experience in the operational area and quality management in the pharmaceutical industry
- 15 years of experience in supply chain management and quality management in various industries
- Studied economic geography at the Ludwig Maximilian University in Munich

Dr. Mathias Schmidt
Head of regulatory Affairs and Qualified Person
- Qualified Person at Apurano Pharmaceuticals GmbH since 2016
- Study of pharmacy and PhD at the Pharmaceutical Institute of the Eberhard Karls University of Tübingen
- Many years of experience as head of medical-scientific departments in the pharmaceutical industry with a focus on phytopharmaceuticals
- Since 2003 self-employed in the field of drug development and approval
- Processing of quality, toxicology, pharmacovigilance and clinical projects
- Expertise in phased plan and safety assessment procedures for herbal medicinal products
- Qualification as “Qualified Person Pharmacovigilance” and “Qualified Person” in the GMP area including quality management
- Extensive publication activity in the field of herbal medicinal products
- Chairman of the scientific board of trustees of the Society for Phytotherapy and member of the scientific committee of the ESCOP
- Focus on medicinal plants with effects against anxiety and depression, as well as endocrine applications and gastrointestinal diseases
- Deployment as an expert for the EU and the countries of Oceania for the quality and safety of use of kava (Piper methysticum)
SCIENTIFIC ADVISORY BOARD
Apurano Pharmaceuticals is also advised by a Scientific Board with considerable chronic pain, neurology and psychiatry research and development experience.
The team’s combined expertise in neurological research, clinical development, production and company building are critical for delivering on the promise of bringing innovative therapies to market to provide relief and hope for the millions of patients affected by neurological and psychiatric conditions.

Prof. Dr. med. Dr. h.c.
Walter Zieglgänsberger
Chairman, chronic pain
- Emeritus Professor of Clinical Neuropharmacology at the Max Planck Institute for Psychiatry in Munich
- Leading scientist in pain and addiction research
- Winner of the Federal Cross of Merit, 1st class
- Twice laureate of the German Pain Prize
- Founding member of various professional societies
- Reviewer and member of the editorial boards of numerous international journals, including Reviewing Editor for Science

Univ.-Prof. Dr. med. Dr. rer. nat.
Thomas R. Tölle
Chronic pain, neurology
- Head of the center for interdisciplinary pain medicine at the Klinikum Rechts der Isar of the Technical University of Munich and head of the clinical-experimental pain research group
- Head of the office of the German Research Association for Neuropathic Pain project of the BMBF and consortium leader of the Rise-uP project of the Innovation Fund of the G-BA.
- Member of the editorial boards of various specialist journals, tutor for the Alexander von Humboldt Foundation and reviewer for national and international research sponsors

Prof. Dr. pharm.
Rudolf Brenneisen
Pharmacology/Pharmacy
- Professor Emeritus University of Bern for Pharmacy
- 40 years of experience in cannabinoid pharmacology
- Board member of the Swiss Society for Medicinal Cannabis and Cannabinoids
- Secretary General of the Swiss Academy of Pharmaceutical Sciences
- Editor-in-Chief of the journal “Medical Cannabis and Cannabinoids”

Prof. Dr. med.
Dan Ziegler
Diabetic polyneuropathy
- Deputy Director of the German Diabetes Center
- Specialist in diabetic neuropathic pain
- Member of expert committees for the development of guidelines of national diabetes societies: DDG: Diabetic Neuropathy and ADA: Consensus Report on Diabetic Neuropathy

Prof. Dr. med.
Stefan Lorenzl
Spasticity, Palliative Care, Parkinson’s Disease
- Head of department neurology and palliative care hospital Agatharied (Ludwig Maximilian University Munich)
- Professor for palliative care at the Paracelsus University in Salzburg
- Focus TOP physician for Parkinson’s disease
- Author of over 100 scientific publications
- More than 200 scientific lectures in the last 5 years
- Member of several professional societies
- Head of the working group on palliative care in the German Society for Neurology together with Prof. Rolke

Prof. Dr. med.
Borwin Bandelow
Anxiety Disorders
- Professor Emeritus Neurology and Psychiatry University of Göttingen
- Author of numerous publications and books
- 2019 Focus TOP physician for Anxiety Disorders
- Founder and Honorary Chairman of the Society for Anxiety Research e.V.
- Listed by the magazine Cicero as one of the most important German-speaking intellectuals

Federal State Pharmacy Director a.D.
Hartmut Reinbold
Pharmacy
- Management of the central pharmacy of the LWL Klinik Dortmund for many years
- Long-standing technical chairman of the Drugs Commission of the Regional Association of Westphalia Lippe (LWL)
- Broad professional experience in pharmacology
- Numerous publications, lectures and author of 8 specialist books
- Lecturer for specialist training in psychiatry and geriatric pharmacy for many years
- Pharmacological training for doctors, pharmacists, nursing staff
- Nationwide implementation of clinical visits in psychiatric institutions
- Workshop management for “Meet the Expert”
- Expert for specific and complex pharmacological questions

Prof. Dr. Beat Lutz
Endocannabinoid System
- Director of the Institute for Physiological Chemistry at the Johannes Gutenberg University of Mainz
- World-renowned expert on the endocannabinoid system
- Author of numerous publications e.g. in Nature, Science and Neuroscience
- Chair of the Gordon Conference “Cannabinoid Functions in the CN: 2011
- Chairman of the DFG research area FOR926 (with Prof. A. Zimmer, Bonn): 2008-2014

PD Dr. Carsten Wotjak
Anxiety Disorders, Endocannabinoid System
- Director Preclinic at Boehringer-Ingelheim
- Expert in anxiety disorder and its pathogenesis
- Expert on the role of the endocannabinoid system in fear and anxiety
- Author of numerous publications in leading international scientific journals
- Previous group leader at the Max Planck Institute for Psychiatry, Stress Neurobiology & Neurogenetic

Prof. Dr. med. Kai G. Kahl
Anxiety Disorders, Affective Disorders
- Executive Senior Physician at the Clinic for Psychiatry, Social Psychiatry and Psychotherapy at the Hannover Medical School
- Medical management of the training institute for behavioral therapy and behavioral medicine (AVVM), supervisor
- Head of the research group “Brain, heart and mental disorders”
- Head of the AGNP working group “Polypharmacy”
- Clinical and scientific expertise in affective and personality disorders. Focus on borderline disorders

Prof. Dr. med.
Michael Kellner
Anxiety and Obsessive–compulsive disorders
- Head of the speciality clinic for anxiety disorder, obsessive-compulsive disorder and post-traumatic stress disorder at the Klinikum rechts der Isar of the Technical University Munich
- Professor at the University Hospital Hamburg-Eppendorf
- Previous dead of department Psychiatry, Psychotherapy & Psychosomatic Medicine at the Herford Clinic (teaching hospital of the Ruhr University Bochum)
- Member of the S3 Guideline Commission for Anxiety Disorders
- 2011-2019 Focus Top Physician for Anxiety Disorder
- Development of the panic and anxiety outpatient clinic at the MPI in Munich
- Establishment of the first German special outpatient clinic for post-traumatic stress disorders in Hamburg
- Author of over 100 international peer-reviewed publications on panic and anxiety disorders, post-traumatic stress disorder, depression, obsessive-compulsive disorder; Focus on psychoneuroendocrinology, psychopharmacology and experimental psychopathology
- Indication areas: anxiety disorders, obsessive-compulsive disorder, post-traumatic stress disorder, depression

Prim. Univ.-Prof. Dr.
Rudolf Likar, MSc
Chronic pain, palliative care
- Head of the department for anaesthesiology, general intensive care medicine, emergency medicine, interdisciplinary pain therapy and palliative medicine at the Klinikum Klagenfurt am Wörthersee and at the LKH Wolfsberg
- Member of the editorial boards of various specialist journals, reviewer for national and international research projects, chair at the Sigmund Freud University in Vienna
- Board member in Austrian professional societies (ÖSG, OPG, ÖGARI)

Dr. Michael Schatman
Chronic pain
- Clinical psychologist with 37 years of experience in multidisciplinary chronic pain therapy
- Faculty member at the Department of Anesthesiology, Perioperative Care, and Pain Medicine and the Division of Medical Ethics at the NYU Grossman School of Medicine
- Author of over 180 journal articles and book chapters on pain management
- Regular speaker at local, national, and international pain management
- Current research focus in the area of neuromodulation
- 10-year tenure as Editor-in-Chief of the Journal of Pain Research
- Editor of the books Ethical Issues in Chronic Pain Management and Chronic Pain Management: Guidelines for Multidisciplinary Program Development
- Former chairperson of the Ethics Special Interest Group of the American Pain Society and the Ethics Committee of the American Academy of Pain Medicine

Smart bio-
nanotechnology
OUR PATENTED MANUFACTURING TECHNOLOGY PuranoTec®
Many plant substances have great therapeutic potential, but are often poorly soluble in water, making them difficult to absorb into the body. But other factors such as the charge and size of drugs as well as their permeability through cell membranes also play a crucial role in absorption. In order to ensure effective absorption, it is important how drugs are “packaged”. The type of packaging also determines how high the bioavailability of the drug is; i.e. how much of the active ingredient is ultimately available unchanged in the bloodstream. Using the patented PuranoTec® manufacturing process, the lipophilic, water-insoluble active ingredients of the plant are enclosed in a carrier that is surrounded by a water-soluble shell, the smart coating. The manufacturing technology enables the particle size of these carriers, also called SmartLipids, to be reduced to < 250 nm and also ensures the physical stability of the particles in aqueous solution. The PuranoTec® manufacturing process converts herbal active ingredients into an aqueous nanodispersion, which, when applied sublingually (as a mouth spray), allows for easy absorption and high bioavailability. PuranoTec® does not use alcoholic solvents at all, so that no irritation is caused when applied to the oral mucosa.
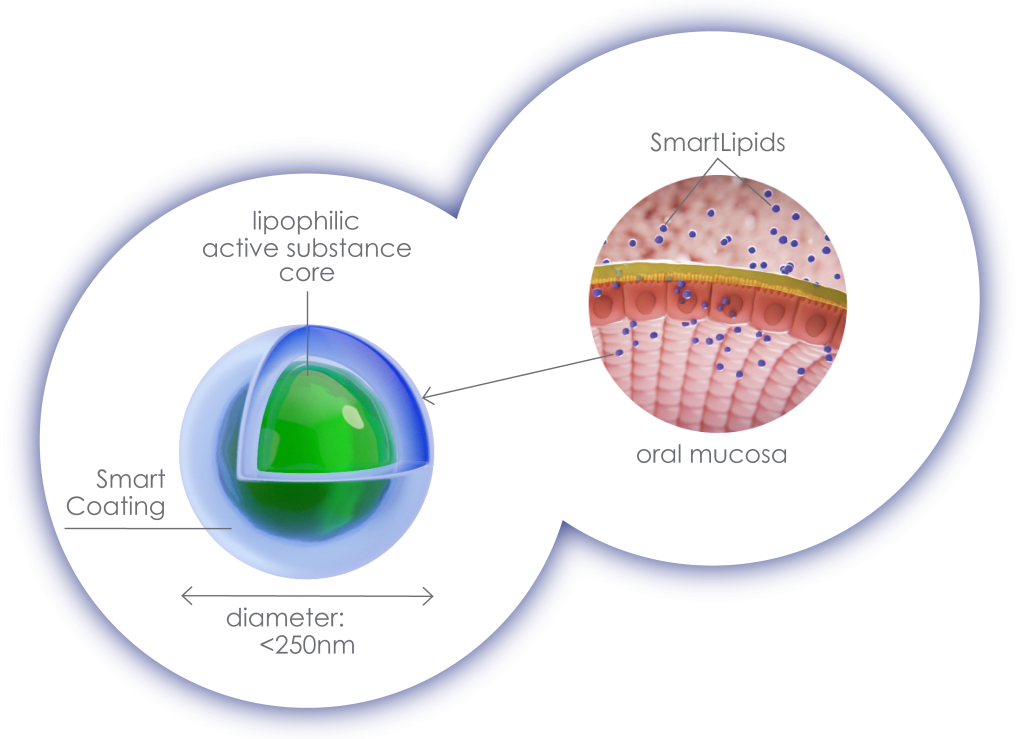
Fig. left: Structure of the SmartLipids using the PuranoTec® manufacturing process Fig. right: Effective absorption of the SmartLipids via the oral mucosa due to their particle size of <250nm and the Smart Coating

PRODUCT
PIPELINE
PIPELINE
Using our patented PuranoTec® manufacturing technology, we develop easy-to-absorb herbal medicinal substances. We are researching various active ingredient candidates for different indications. Our current focus is on the development and clinical testing of PuranoTec®-based cannabinoid formulations. While our CBD-focused active ingredient Beleronap (investigational drug designation AP703) is still going through the preclinical stage, our THC-focused active ingredient Adezunap (investigational drug designation AP707) already has EMA approval to conduct the DISCOVER approval studies. The Phase III clinical trial for Adezunap will start at the end of 2023 in the indications listed below.
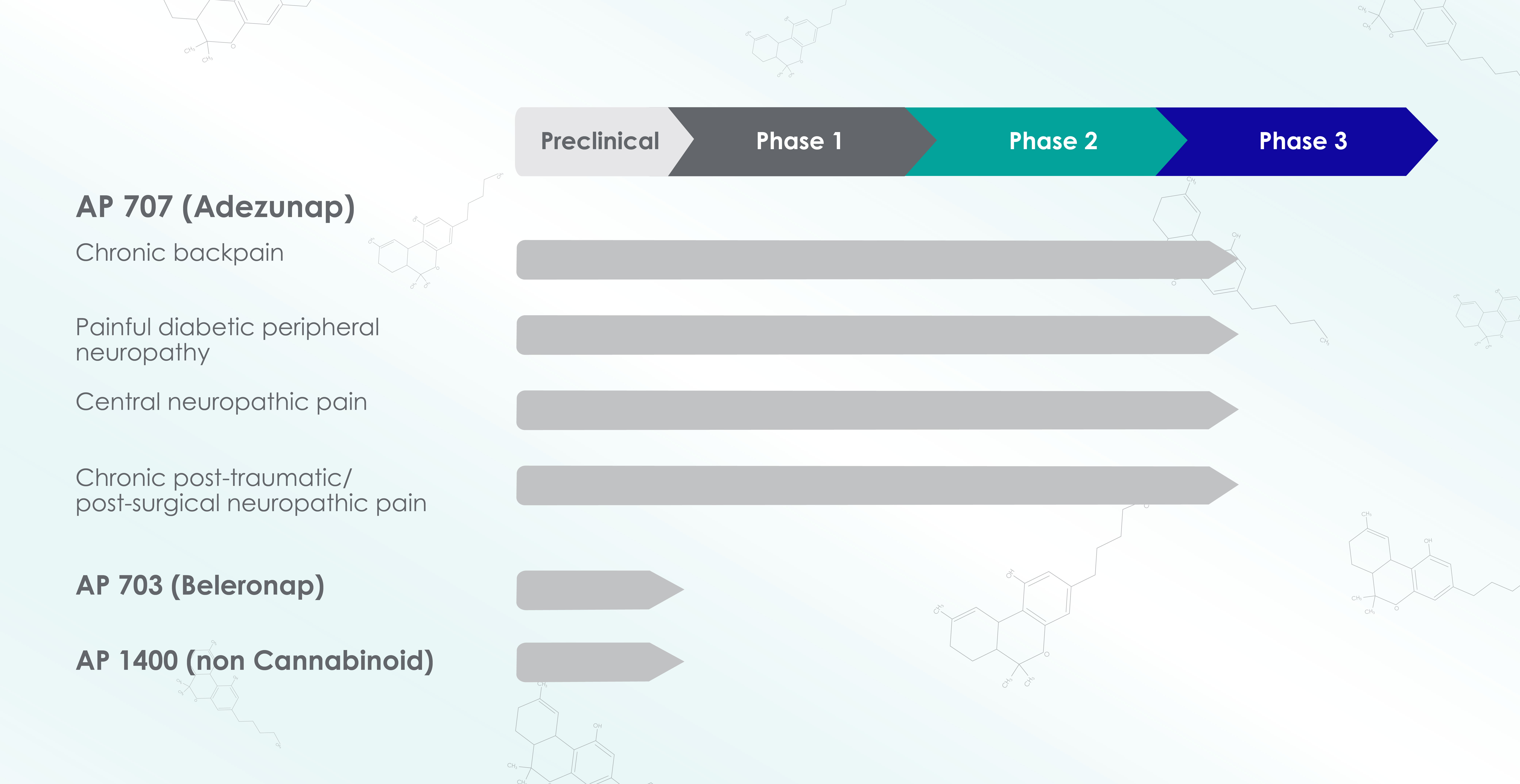
Fig. Drug pipeline of Apurano Pharmaceuticals

Indications
APURANO’S MANUFACTURING TECHNOLOGY PURANOTEC® FOR CHRONIC PAIN
1.7 billion people worldwide suffer from chronic pain. 78% of chronic pain patients are dissatisfied with their current therapy because it does not sufficiently relieve their pain. Cannabinoids represent a therapeutic alternative or useful supplement to standard pain treatment. Although these are promising for chronic pain due to their analgesic effect, there is currently a lack of suitable pharmaceutical formulations and dosage forms.
This is exactly where we at Apurano come in!
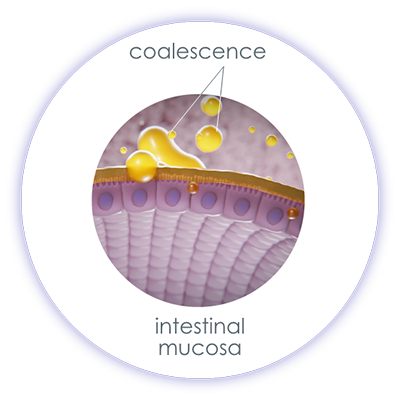
ABSORPTION OF CURRENT CANNABINOID FORMULATIONS
As with many herbal medicines, cannabinoids are lipophilic, almost water-insoluble substances that are difficult to absorb in the watery environment of our body. This is due, among other things, to the nature of our mucous membranes (mucosa). The top layer of every mucosa in our body is the mucin layer. This consists of 95% water and 5% glycoproteins and represents the first absorption barrier for active ingredients. In addition, the mucous membrane itself acts like a filter that only allows drops and particles to pass through if they are small enough. For example, the oral mucosa has an absorption limit of 0.4 µm, the intestinal mucosa of approx. 5 µm. If oily cannabis formulations (e.g. dronabinol or THC-containing cannabis extracts) come into contact with the aqueous mucin layer, this leads to the drops flowing together (coalescence), so that the active ingredient is only insufficiently absorbed due to the drop size.
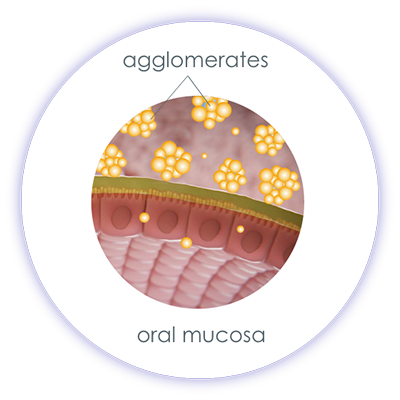
If a cannabis formulation in which the ∆-9-THC is dissolved in ethanol comes into contact with water, the ∆-9-THC dissolved in ethanol precipitates and forms agglomerates. This results in large particles that cannot be absorbed through the oral mucosa. Thus, different drug formulations of the same active ingredient can significantly influence its absorption and bioavailability.
ADEZUNAP: INNOVATIVE NANO-ECS-MODULATOR
With our active ingredient ADEZUNAP, the cannabis flower is processed using the patented PuranoTec® manufacturing process so that the cannabinoids (primarily ∆-9-THC) are available as an aqueous nanodispersion. The dispersion is applied sublingually as a mouth spray and the cannabinoids are absorbed quickly and effectively via the oral mucosa. This is possible because the lipophilic, water-insoluble cannabinoids are enclosed in a carrier that is surrounded by a water-soluble shell, the smart coating. As so-called SmartLipids, they remain physically stable in an aqueous environment (no coalescence or agglomerate formation), which ensures passage through the aqueous mukin layer. On the other hand, due to the small particle size of <250 nm, they can enter the bloodstream directly via the oral mucosa.

Fig. left: Structure of the SmartLipids using the PuranoTec® manufacturing process Fig. right: Effective absorption of the SmartLipids via the oral mucosa due to their particle size of <250nm and the Smart Coating
APURANO’S MANUFACTURING TECHNOLOGY PURANOTEC® FOR PSYCHATRIC DISEASES
In addition to our active ingredient Adezunap, which will be used to treat chronic pain once approved, we are developing another cannabinoid active ingredient in parallel: BELERONAP. The CBD-focused active ingredient Beleronap is also produced using our patented PuranoTec® manufacturing process and applied sublingually as a mouth spray. The therapeutic area of application for Beleronap will primarily include psychiatric illnesses.

professional
circles
PROFESSIONAL GROUPS
INFORMATIONEN FOR MEDICAL PROFESSIONALS
If you do not have a user account yet, please register using the link below. After your data and access authorization have been checked, you will receive an e-mail with a link with which you can activate your access data.
If you are already registered and have activated your access, you can now log in here:

QLUMBUS
App
QLUMBUS App
Subjects and medical staff of the DISCOVER studies can access the Qlumbus web app at this LINK. Subjects can access the Qlumbus smartphone app by scanning the following QR codes:
QR-Code Playstore

QR-Code APPstore


CAREER
WE LOOK FORWARD TO RECEIVING YOUR APPLICATION
Please send your application to the person named in the job profile.

NEWS
NEWS
In our news area we keep you up to date on current events and findings related to our therapeutic areas.

CONTACT
CONTACT FORM
If you have any questions about our products, please contact us!
We will immediately contact you.
 | E-mail info@apurano.de |  | Phone Order +49 8024 46869 80 Advice Hotline +49 8024 46869 70 Hotline Qlumbus-App DISCOVER +49 8024 46869 90 Fax +49 8024 46869 99 |
 | Address Apurano Pharmaceuticals GmbH Birkerfeld 12 | 83627 Warngau | Germany |